What is the number of valence electrons in carbon?
Oxygen is in the 16th group of the periodic table. It has 6 electrons in its valence shell with a main valence number of 2. Chlorine Carbon is in the 17th group of the periodic table.
- The carbon cycle shows how carbon moves through the living and non-living parts of the environment. Octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons (has some exceptions).
- For example, carbon has a valence of 4 and has 4 valence electrons, nitrogen has a valence of 3 and has 5 valence electrons, and oxygen has a valence of 2 and has 6 valence electrons. Hydrogen is an important special case with a single valence electron and a valence of 1.
- Carbon is a nonmetal in group 14 of the periodic table. Like other group 14 elements, carbon has four valence electrons. In a covalent bond, two atoms share a pair of electrons. By forming four covalent bonds, carbon shares four pairs of electrons, thus filling its outer energy level and achieving stability.
1 Answer
Explanation:
Carbon has six electrons in its neutral state.
6 electrons - 2 electrons in the first energy level = 4 valance electrons
Valance electrons are the electrons in the outermost energy level.
The first two electrons go into the 1 s orbital the lowest energy state.
which fills the first energy level. These two electrons will not be valance electrons.
The next two electrons go into the 2 s orbital the lowest energy level in the second valance shell. These are valance electrons.
The next two electrons go into the 2 p orbital the highest energy level in the second valance. These are valance electrons.
Often Carbon will hybridize the four valance electrons into 4 equal new orbitals 4 sp3 orbitals. In the hybridized state carbon can form four bonds such as CO2
In the lowest energy state or ground state Carbon can form 2 bonds using only the 2 p orbitals, such as CO
Related questions
The Chemical Basis for Life
Carbon is the most important element to living things because it can form many different kinds of bonds and form essential compounds.
Learning Objectives
Explain the properties of carbon that allow it to serve as a building block for biomolecules
Key Takeaways
Key Points
- All living things contain carbon in some form.
- Carbon is the primary component of macromolecules, including proteins, lipids, nucleic acids, and carbohydrates.
- Carbon’s molecular structure allows it to bond in many different ways and with many different elements.
- The carbon cycle shows how carbon moves through the living and non-living parts of the environment.
Key Terms
- octet rule: A rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons (has some exceptions).
- carbon cycle: the physical cycle of carbon through the earth’s biosphere, geosphere, hydrosphere, and atmosphere; includes such processes as photosynthesis, decomposition, respiration and carbonification
- macromolecule: a very large molecule, especially used in reference to large biological polymers (e.g., nucleic acids and proteins)
Carbon is the fourth most abundant element in the universe and is the building block of life on earth. On earth, carbon circulates through the land, ocean, and atmosphere, creating what is known as the Carbon Cycle. This global carbon cycle can be divided further into two separate cycles: the geological carbon cycles takes place over millions of years, whereas the biological or physical carbon cycle takes place from days to thousands of years. In a nonliving environment, carbon can exist as carbon dioxide (CO2), carbonate rocks, coal, petroleum, natural gas, and dead organic matter. Plants and algae convert carbon dioxide to organic matter through the process of photosynthesis, the energy of light.
Carbon is present in all life: All living things contain carbon in some form, and carbon is the primary component of macromolecules, including proteins, lipids, nucleic acids, and carbohydrates. Carbon exists in many forms in this leaf, including in the cellulose to form the leaf’s structure and in chlorophyll, the pigment which makes the leaf green.
Carbon is Important to Life
In its metabolism of food and respiration, an animal consumes glucose (C6H12O6), which combines with oxygen (O2) to produce carbon dioxide (CO2), water (H2O), and energy, which is given off as heat. The animal has no need for the carbon dioxide and releases it into the atmosphere. A plant, on the other hand, uses the opposite reaction of an animal through photosynthesis. It intakes carbon dioxide, water, and energy from sunlight to make its own glucose and oxygen gas. The glucose is used for chemical energy, which the plant metabolizes in a similar way to an animal. The plant then emits the remaining oxygen into the environment.
Cells are made of many complex molecules called macromolecules, which include proteins, nucleic acids (RNA and DNA), carbohydrates, and lipids. The macromolecules are a subset of organic molecules (any carbon-containing liquid, solid, or gas) that are especially important for life. The fundamental component for all of these macromolecules is carbon. The carbon atom has unique properties that allow it to form covalent bonds to as many as four different atoms, making this versatile element ideal to serve as the basic structural component, or “backbone,” of the macromolecules.
Structure of Carbon
Individual carbon atoms have an incomplete outermost electron shell. With an atomic number of 6 (six electrons and six protons), the first two electrons fill the inner shell, leaving four in the second shell. Therefore, carbon atoms can form four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: it has the chemical formula CH4. Each of its four hydrogen atoms forms a single covalent bond with the carbon atom by sharing a pair of electrons. This results in a filled outermost shell.
Structure of Methane: Methane has a tetrahedral geometry, with each of the four hydrogen atoms spaced 109.5° apart.
Hydrocarbons
Hydrocarbons are important molecules that can form chains and rings due to the bonding patterns of carbon atoms.
Learning Objectives
Discuss the role of hydrocarbons in biomacromolecules
Key Takeaways
Key Points
- Hydrocarbons are molecules that contain only carbon and hydrogen.
- Due to carbon’s unique bonding patterns, hydrocarbons can have single, double, or triple bonds between the carbon atoms.
- The names of hydrocarbons with single bonds end in “-ane,” those with double bonds end in “-ene,” and those with triple bonds end in “-yne”.
- The bonding of hydrocarbons allows them to form rings or chains.
Key Terms
- covalent bond: A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons.
- aliphatic: Of a class of organic compounds in which the carbon atoms are arranged in an open chain.
- aromatic: Having a closed ring of alternate single and double bonds with delocalized electrons.
Hydrocarbons
Hydrocarbons are organic molecules consisting entirely of carbon and hydrogen, such as methane (CH4). Hydrocarbons are often used as fuels: the propane in a gas grill or the butane in a lighter. The many covalent bonds between the atoms in hydrocarbons store a great amount of energy, which is released when these molecules are burned (oxidized). Methane, an excellent fuel, is the simplest hydrocarbon molecule, with a central carbon atom bonded to four different hydrogen atoms. The geometry of the methane molecule, where the atoms reside in three dimensions, is determined by the shape of its electron orbitals. The carbon and the four hydrogen atoms form a shape known as a tetrahedron, with four triangular faces; for this reason, methane is described as having tetrahedral geometry.
Methane: Methane has a tetrahedral geometry, with each of the four hydrogen atoms spaced 109.5° apart.
As the backbone of the large molecules of living things, hydrocarbons may exist as linear carbon chains, carbon rings, or combinations of both. Furthermore, individual carbon-to-carbon bonds may be single, double, or triple covalent bonds; each type of bond affects the geometry of the molecule in a specific way. This three-dimensional shape or conformation of the large molecules of life (macromolecules) is critical to how they function.
Hydrocarbon Chains
Hydrocarbon chains are formed by successive bonds between carbon atoms and may be branched or unbranched. The overall geometry of the molecule is altered by the different geometries of single, double, and triple covalent bonds. The hydrocarbons ethane, ethene, and ethyne serve as examples of how different carbon-to-carbon bonds affect the geometry of the molecule. The names of all three molecules start with the prefix “eth-,” which is the prefix for two carbon hydrocarbons. The suffixes “-ane,” “-ene,” and “-yne” refer to the presence of single, double, or triple carbon-carbon bonds, respectively. Thus, propane, propene, and propyne follow the same pattern with three carbon molecules, butane, butene, and butyne for four carbon molecules, and so on. Double and triple bonds change the geometry of the molecule: single bonds allow rotation along the axis of the bond, whereas double bonds lead to a planar configuration and triple bonds to a linear one. These geometries have a significant impact on the shape a particular molecule can assume.
Hydrocarbon Chains: When carbon forms single bonds with other atoms, the shape is tetrahedral. When two carbon atoms form a double bond, the shape is planar, or flat. Single bonds, like those found in ethane, are able to rotate. Double bonds, like those found in ethene cannot rotate, so the atoms on either side are locked in place.
Hydrocarbon Rings
The hydrocarbons discussed so far have been aliphatic hydrocarbons, which consist of linear chains of carbon atoms. Another type of hydrocarbon, aromatic hydrocarbons, consists of closed rings of carbon atoms. Ring structures are found in hydrocarbons, sometimes with the presence of double bonds, which can be seen by comparing the structure of cyclohexane to benzene. The benzene ring is present in many biological molecules including some amino acids and most steroids, which includes cholesterol and the hormones estrogen and testosterone. The benzene ring is also found in the herbicide 2,4-D. Benzene is a natural component of crude oil and has been classified as a carcinogen. Some hydrocarbons have both aliphatic and aromatic portions; beta-carotene is an example of such a hydrocarbon.
Hydrocarbon Rings: Carbon can form five-and six membered rings. Single or double bonds may connect the carbons in the ring, and nitrogen may be substituted for carbon.
Organic Isomers
Isomers are molecules with the same chemical formula but have different structures, which creates different properties in the molecules.
Key Takeaways
Key Points
- Isomers are molecules with the same chemical formula but have different structures.
- Isomers differ in how their bonds are positioned to surrounding atoms.
- When the carbons are bound on the same side of the double bond, this is the cis configuration; if they are on opposite sides of the double bond, it is a trans configuration.
- Triglycerides, which show both cis and trans configurations, can occur as either saturated or unsaturated, depending upon how many hydrogen atoms they have attached to them.
Key Terms
- fatty acid: Any of a class of aliphatic carboxylic acids, of general formula CnH2n+1COOH, that occur combined with glycerol as animal or vegetable oils and fats.
- isomer: Any of two or more compounds with the same molecular formula but with different structure.
The three-dimensional placement of atoms and chemical bonds within organic molecules is central to understanding their chemistry. Molecules that share the same chemical formula but differ in the placement (structure) of their atoms and/or chemical bonds are known as isomers.
Structural Isomers
Structural isomers (such as butane and isobutane ) differ in the placement of their covalent bonds. Both molecules have four carbons and ten hydrogens (C4H10), but the different arrangement of the atoms within the molecules leads to differences in their chemical properties. For example, due to their different chemical properties, butane is suited for use as a fuel for cigarette lighters and torches, whereas isobutane is suited for use as a refrigerant and a propellant in spray cans.
Geometric Isomers
Geometric isomers, on the other hand, have similar placements of their covalent bonds but differ in how these bonds are made to the surrounding atoms, especially in carbon-to-carbon double bonds. In the simple molecule butene (C4H8), the two methyl groups (CH3) can be on either side of the double covalent bond central to the molecule. When the carbons are bound on the same side of the double bond, this is the cis configuration; if they are on opposite sides of the double bond, it is a trans configuration. In the trans configuration, the carbons form a more or less linear structure, whereas the carbons in the cis configuration make a bend (change in direction) of the carbon backbone.
Isomers: Molecules that have the same number and type of atoms arranged differently are called isomers. (a) Structural isomers have a different covalent arrangement of atoms. (b) Geometric isomers have a different arrangement of atoms around a double bond. (c) Enantiomers are mirror images of each other.
Cis or Trans Configurations
Cis and Trans Fatty Acids: These space-filling models show a cis (oleic acid) and a trans (eliadic acid) fatty acid. Notice the bend in the molecule cause by the cis configuration.
In triglycerides (fats and oils), long carbon chains known as fatty acids may contain double bonds, which can be in either the cis or trans configuration. Fats with at least one double bond between carbon atoms are unsaturated fats. When some of these bonds are in the cis configuration, the resulting bend in the carbon backbone of the chain means that triglyceride molecules cannot pack tightly, so they remain liquid (oil) at room temperature. On the other hand, triglycerides with trans double bonds (popularly called trans fats), have relatively linear fatty acids that are able to pack tightly together at room temperature and form solid fats.
In the human diet, trans fats are linked to an increased risk of cardiovascular disease, so many food manufacturers have reduced or eliminated their use in recent years. In contrast to unsaturated fats, triglycerides without double bonds between carbon atoms are called saturated fats, meaning that they contain all the hydrogen atoms available. Saturated fats are a solid at room temperature and usually of animal origin.
Organic Enantiomers
Enantiomers share the same chemical structure and bonds but differ in the placement of atoms such that they are mirror images of each other.
Key Takeaways
Key Points

- Enantiomers are stereoisomers, a type of isomer where the order of the atoms in the two molecules is the same but their arrangement in space is different.
- Many molecules in the bodies of living beings are enantiomers; there is sometimes a large difference in the effects of two enantiomers on organisms.
- Enantiopure compounds refer to samples having, within the limits of detection, molecules of only one chirality.
- Compounds that are enantiomers of each other have the same physical properties except for the direction in which they rotate polarized light and how they interact with different optical isomers of other compounds.
Key Terms
- enantiomer: One of a pair of stereoisomers that is the mirror image of the other, but may not be superimposed on this other stereoisomer.
- chirality: The phenomenon in chemistry, physics, and mathematics in which objects are mirror images of each other, but are not identical.
- stereoisomer: one of a set of the isomers of a compound in which atoms are arranged differently about a chiral center; they exhibit optical activity
Enantiomers
Stereoisomers are a type of isomer where the order of the atoms in the two molecules is the same but their arrangement in space is different. The two main types of stereoisomerism are diastereomerism (including ‘cis-trans isomerism’) and optical isomerism (also known as ‘enantiomerism’ and ‘chirality’). Optical isomers are stereoisomers formed when asymmetric centers are present; for example, a carbon with four different groups bonded to it. Enantiomers are two optical isomers (i.e. isomers that are reflections of each other). Every stereocenter in one isomer has the opposite configuration in the other. They share the same chemical structure and chemical bonds, but differ in the three-dimensional placement of atoms so that they are mirror images, much as a person’s left and right hands are. Compounds that are enantiomers of each other have the same physical properties except for the direction in which they rotate polarized light and how they interact with different optical isomers of other compounds.
The amino acid alanine is example of an entantiomer. The two structures, D-alanine and L-alanine, are non-superimposable. In nature, only the L-forms of amino acids are used to make proteins. Some D forms of amino acids are seen in the cell walls of bacteria, but never in their proteins. Similarly, the D-form of glucose is the main product of photosynthesis and the L-form of the molecule is rarely seen in nature.
Enantiomers: D-alanine and L-alanine are examples of enantiomers or mirror images. Only the L-forms of amino acids are used to make proteins.
Organic compounds that contain a chiral carbon usually have two non-superposable structures. These two structures are mirror images of each other and are, thus, commonly called enantiomorphs; hence, this structural property is now commonly referred to as enantiomerism. Enantiopure compounds refer to samples having, within the limits of detection, molecules of only one chirality.
Enantiomers of each other often show different chemical reactions with other substances that are also enantiomers. Since many molecules in the bodies of living beings are enantiomers themselves, there is sometimes a marked difference in the effects of two enantiomers on living beings. In drugs, for example, often only one of a drug’s enantiomers is responsible for the desired physiologic effects, while the other enantiomer is less active, inactive, or sometimes even responsible for adverse effects. Owing to this discovery, drugs composed of only one enantiomer (“enantiopure”) can be developed to enhance the pharmacological efficacy and sometimes do away with some side effects.
Organic Molecules and Functional Groups
Functional groups are groups of molecules attached to organic molecules and give them specific identities or functions.

Learning Objectives
Describe the importance of functional groups to organic molecules
Key Takeaways
Key Points
- Functional groups are collections of atoms that attach the carbon skeleton of an organic molecule and confer specific properties.
- Each type of organic molecule has its own specific type of functional group.
- Functional groups in biological molecules play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
- Functional groups include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl.
Key Terms
- hydrophobic: lacking an affinity for water; unable to absorb, or be wetted by water
- hydrophilic: having an affinity for water; able to absorb, or be wetted by water
Location of Functional Groups
Functional groups are groups of atoms that occur within organic molecules and confer specific chemical properties to those molecules. When functional groups are shown, the organic molecule is sometimes denoted as “R.” Functional groups are found along the “carbon backbone” of macromolecules which is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen. Molecules with other elements in their carbon backbone are substituted hydrocarbons. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.

Properties of Functional Groups
A functional group can participate in specific chemical reactions. Some of the important functional groups in biological molecules include: hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play an important role in the formation of molecules like DNA, proteins, carbohydrates, and lipids.
Classifying Functional Groups
Functional groups are usually classified as hydrophobic or hydrophilic depending on their charge or polarity. An example of a hydrophobic group is the non-polar methane molecule. Among the hydrophilic functional groups is the carboxyl group found in amino acids, some amino acid side chains, and the fatty acid heads that form triglycerides and phospholipids. This carboxyl group ionizes to release hydrogen ions (H+) from the COOH group resulting in the negatively charged COO– group; this contributes to the hydrophilic nature of whatever molecule it is found on. Other functional groups, such as the carbonyl group, have a partially negatively charged oxygen atom that may form hydrogen bonds with water molecules, again making the molecule more hydrophilic.
Examples of functional groups: The functional groups shown here are found in many different biological molecules, where “R” is the organic molecule.
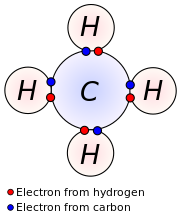
Four Carbon Valence Electrons
Hydrogen Bonds between Functional Groups
Hydrogen bonds between functional groups (within the same molecule or between different molecules) are important to the function of many macromolecules and help them to fold properly and maintain the appropriate shape needed to function correctly. Hydrogen bonds are also involved in various recognition processes, such as DNA complementary base pairing and the binding of an enzyme to its substrate.
Carbon Valence Electrons Model
Hydrogen bonds in DNA: Hydrogen bonds connect two strands of DNA together to create the double-helix structure.
