Every spring, millions of Americans file their income tax forms. The different rules determine how much tax a person pays. There are also exceptions to the rules. You pay less tax if you are married and/or have children. There are certain limits on how much money you can make before paying taxes. The rule is that you pay taxes, but there are also exceptions based on your personal situation. The bonding rules for molecules are generally applicable, but there are some exceptions allowed.
Exceptions to the Octet Rule
As the saying goes, all rules are made to be broken. When it comes to the octet rule, this is true. Exceptions to the octet rule fall into one of three categories: (1) an incomplete octet, (2) odd-electron molecules, and (3) an expanded octet.
Incomplete Octet
If you are asking for the four quantum numbers of an aluminum valence electron, note that the valence shell for Al is 3s2 3p1. The principal quantum number (n) has a. Aluminum number of valence electrons. If you have any questions or good suggestions on our products and site, or if you want to know more information about our products, please write them and send to us, we will contact you within one business day. Updated 6 months ago Author has 174 answers and 26.8K answer views If you are asking for the four quantum numbers of an aluminum valence electron, note that the valence shell for Al is 3s2 3p1. The principal quantum number (n) has a value of 0, 1, 2, 3, 4,. For Al, the principal quantum number for the valence shell electron is 3.
In some compounds, the number of electrons surrounding the central atom in a stable molecule is fewer than eight. Beryllium is an alkaline earth metal and so may be expected to form ionic bonds. However, its very small size and somewhat higher ionization energy compared to other metals actually leads beryllium to form primarily molecular compounds. Since beryllium only has two valence electrons, it does not typically attain an octet through sharing of electrons. The Lewis structure of gaseous beryllium hydride (left( ce{BeH_2} right)) consists of two single covalent bonds between (ce{Be}) and (ce{H}) (see figure below).
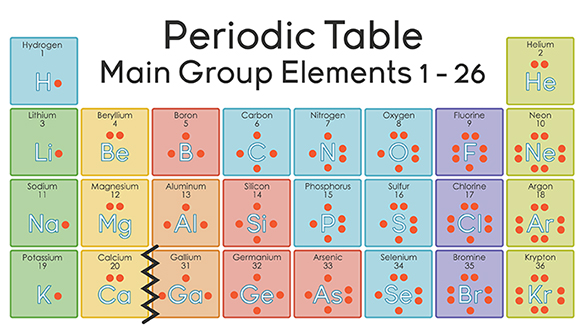
Boron and aluminum, with three valence electrons, also tend to form covalent compounds with an incomplete octet. The central boron atom in boron trichloride (left( ce{BCl_3} right)) has six valence electrons, as shown in the figure below.
Odd-Electron Molecules
Valence Electrons In Aluminum
There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in such a molecule to satisfy the octet rule. An example is nitrogen dioxide (left( ce{NO_2} right)). Each oxygen atom contributes six valence electrons and the nitrogen atom contributes five for a total of seventeen. The Lewis structure for (ce{NO_2}) appears in the figure below.
Number Of Valence Electrons In Aluminum Atom
Expanded Octets
Atoms of the second period cannot have more than eight valence electrons around the central atom. However, atoms of the third period and beyond are capable of exceeding the octet rule by having more than eight electrons around the central atom. Starting with the third period, the (d) sublevel becomes available, so it is possible to use these orbitals in bonding, resulting in an expanded octet.
Phosphorus and sulfur are two elements that react with halogen elements and make stable compounds with expanded octets. In phosphorus pentachloride, the central phosphorus atom makes five single bonds to chlorine atoms and, as a result, has ten electrons surrounding it (see figure below). In sulfur hexafluoride, the central sulfur atom has twelve electrons from its six bonds to fluorine atoms (see figure below).
Summary
- Exceptions exist to the rules for covalent bonding.
- These exceptions apply to atoms whose electrons will not accommodate the normal octet rule.
Contributors and Attributions
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.

Valence Electrons
Key Questions
The valence electrons are the electrons that determine the most typical bonding patterns for an element.
These electrons are found in the s and p orbitals of the highest energy level for the element.
Sodium
#1s^2 2s^2 2p^6 3s^1#
Sodium has 1 valence electron from the 3s orbitalPhosphorus
#1s^2 2s^2 2p^6 3s^2 3p^3#
Phosphorus has 5 valence electrons 2 from the 3s and 3 from the 3pLets take the ionic formula for Calcium Chloride is
#CaCl_2# Calcium is an Alkaline Earth Metal in the second column of the periodic table. This means that calcium
#s^2# has 2 valence electrons it readily gives away in order to seek the stability of the octet. This makes calcium a Ca+2 cation.Chlorine is a Halogen in the 17th column or
#s^2p^5# group.
Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. This makes chlorine a#Cl^(−1)# anion.Ionic bonds form when the charges between the metal cation and non-metal anion are equal and opposite. This means that two
#Cl^(−1)# anions will balance with one#Ca^(+2)# cation.This makes the formula for calcium chloride,
#CaCl_2# .For the example Aluminum Oxide
#Al_2O_3# Aluminum
#s^2p^1# has 3 valence electrons and an oxidation state of +3 or#Al^(+3)#
Oxygen#s^2p^4# has 6 valence electrons and an oxidation state of -2 or#O^(−2)# The common multiple of 2 and 3 is 6.
We will need 2 aluminum atoms to get a +6 charge and 3 oxygen atoms to get a -6 charge. When the charges are equal and opposite the atoms will bond as#Al_2O_3# .In molecular (covalent) compounds these same valence electrons are shared by atoms in order to satisfy the rule of octet.
I hope this is helpful.
SMARTERTEACHERAnswer:
The number of electrons in an atom's outermost valence shell governs its bonding behaviour.
Explanation:
The valence electrons are the electrons in the outermost electron shell of an atom.
That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.
Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration
#s^2p^6# .This tendency is called the octet rule, because the bonded atoms have eight valence electrons.
METALS
The most reactive kind of metallic element is a metal from Group 1 (e.g., sodium or potassium).
An atom in Group 1 has only a single valence electron. This one valence electron is easily lost to form a positive ion with an
#s^2p^6# configuration (e.g.,#'Na'^+# or#'K'^+# ).A metal from Group 2 (e.g., magnesium) is somewhat less reactive, because each atom must lose two valence electrons to form a positive ion (e.g.,
#'Mg'^(2+)# with an#s^2p^6# configuration.Within each group of metals, reactivity increases as you go down the group.
The valence electrons are less tightly bound and easier to remove, because they are farther away from the nucleus of the atom.
NONMETALS
A nonmetal tends to attract additional valence electrons to attain a full valence shell.
It can either share electrons with a neighboring atom to form a covalent bond or it can remove electrons from another atom to form an ionic bond.
The most reactive kind of nonmetal is a halogen such as fluorine or chlorine.
It has an
#s^2p^5# electron configuration, so it requires only one additional valence electron to form a closed shell.To form an ionic bond, a halogen atom can remove an electron from another atom in order to form an anion (e.g.,
#'F'^'-', 'Cl'^'-'# , etc.).To form a covalent bond, one electron from the halogen and one electron from another atom form a shared pair (e.g., in
#'H–F'# , the dash represents a shared pair of valence electrons, one from#'H'# and one from#'F'# ).Within each Group of nonmetals, reactivity decreases from top to bottom, because the valence electrons are at progressively higher energies and the atoms do not gain much stability by gaining electrons.
In fact, oxygen (the lightest element in Group 16) is more reactive than chlorine, even though it is not a halogen, because the valence electrons of oxygen are closer to the nucleus (at a lower energy).
Therefore, a metal from the bottom of Group 1 (like potassium) and a nonmetal from the top of Group 17 (like fluorine) will react violently, because they both benefit greatly from the reaction.
#'K'# loses one electron to#'F'# and forms the ionic compound potassium fluoride,#'K'^'+'F'^'-'# .
Questions
